In order to generate patient-derived BC, LC organoids, patients from Santa Maria della Misericordia Hospital-Perugia affected by invasive breast carcinoma or lung adenocarcinoma (LUAD) will be recruited and, immediately after the surgical resection of their treatment-naïve breast tissues, tumor and normal-appearing adjacent-to-tumor (NAT) specimens will be collected. We aim to recruit at least 10 patients from each tumor sub-type. The size of tumor tissue dedicated t organoids generation will be around 1–2 cm3 and contiguous to the diagnostic tissue. Resected normal-appearing adjacent-to-tumor tissue will be obtained from tumor-free surgical margins and its morphology will be controlled by pathologists.

WP1, Study design of 3D culture systems in immune-mediated diseases and cancer
Task 1.1 – 1.2 Establishing a Collection of Patient-Derived BC, LC Organoids
Task 1.3 Establishing a Collection of Patient-Derived SS Organoids
Patients will be consecutively enrolled among subjects undergoing minor salivary gland (MSG) biopsy procedure in the diagnostic work-up of suspected SS. The procedure is minimally invasive, performed in an outpatient clinic, under local anaesthesia. It requires a small incision of the oral mucosa, usually at the lower ip, where one or more MSGs are harvested. Usually, one or two absorbable sutures are placed to accelerate healing. If, following the biopsy procedure, extra tissue not required for diagnostic purposes is available, it will be used for the scope of this study. Subjects fulfilling 2016 ACR/EULAR classification criteria for SS will be included in the patients group. The other subjects will be included as controls, provided none of the exclusion criteria are met.
Exclusion criteria:
- Age < 18 years
- Pregnancy or lactation
- Evidence of HCV infection
- History of radiation of the head and neck
- Diagnosis of other systemic autoimmune diseases
Because this is a pilot study, no sample size calculation is required. We expect to include a total of 40 subjects, of which at least 15 SS patients and 15 controls.
Task 1.4 Establishing a Collection of Patient-Derived MEL Organoids
In order to generate patient-derived MEL organoids, canine melanocytic neoplasms from mucosal sites and their surrounding healthy tissue will beaseptically collected and processed within 2 h from surgical excision performed by surgeons of the University of Perugia Veterinary Teaching Hospital or by veterinary practitioners. Inclusion criteria for the study will be a previous histologic or cytologic diagnosis of melanocytic tumor and a margin of healthy mucosa of atleast 1 cm from the tumor, as detected from the macroscopic examination. Tumors with severe ulceration/necrosis will be excluded from the study. We expect to include a total of 8 subjects.
Task 1.5 Establishing a Collection of Patient-Derived AD Organoids
In order to generate patient-derived AD organoids, both canine lesional and healthy skin biopsies dedicated to routine diagnosis will be aseptically collected by surgeons of the University of Perugia Veterinary Teaching Hospital or by veterinary practitioners and processed within 2 h from surgical excision. The sample will be divided into two parts. One part will be placed in neutral buffered formalin for histological and immunohistochemical investigation, the second will be used to generate organoids. We expect to include a total of 8 subjects.
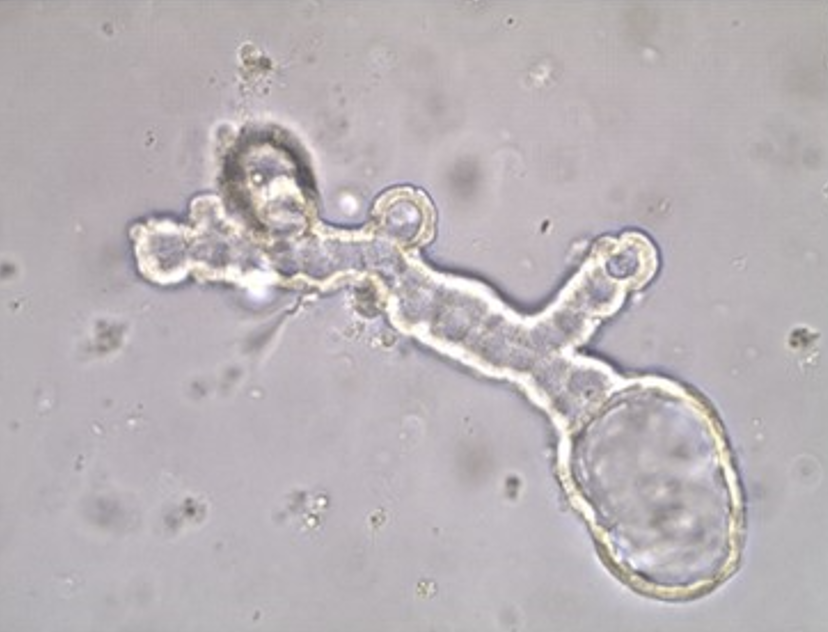
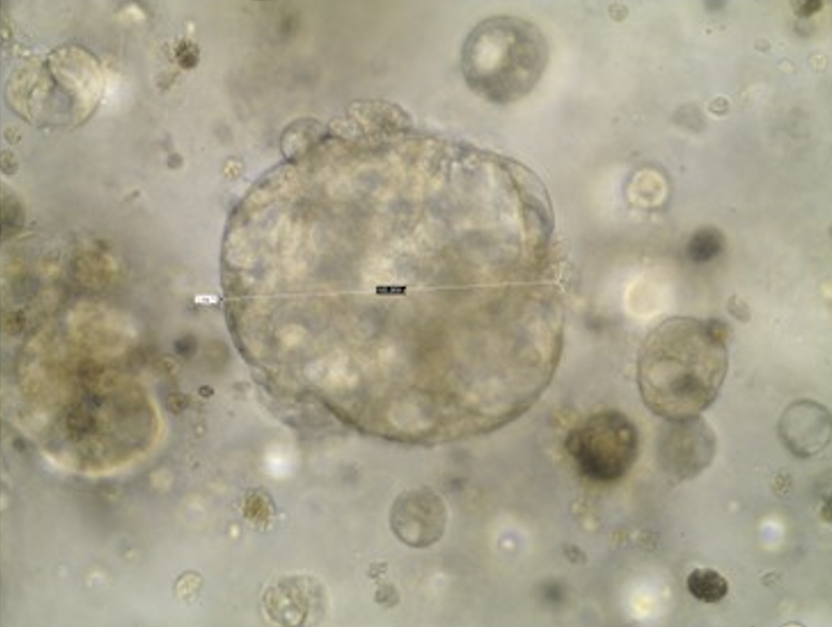
Task 2 Organoid Culture
For organoid generation, tissues will be finely minced, (if available a part of the tissues will be snap frozen and stored at -80°C as back-up) and processed for the isolation of viable cells. Enzymatic digestion of minced tissues with collagenase/dispase/DTT buffer (depending on the type of tissue) for 1–2 h at 37° C will be followed by elimination of red blood cells using ammonium chloride solution. Isolated cell clusters will be resuspended in growth factor reduced solubilized basement membrane (Matrigel®, Corning), to mimic the tumor ECM, and plated into drops in 24-well plates.
Task 3 Organization of the ‘mini-lab organomicrolife’
A ‘mini-lab organomicrolife’ platform will be organized at the Department of Medicine and Surgery, Building D, third floor. A laboratory space (W 6 x L 7,8 meter) is already available and partial equipped with two central laboratory benches, chemical hood, optical microscope (Nikon) and small instruments (such as analytical balance, autoclave and sonicator). Therefore, to complete the cell culture laboratory for organoids development we will need to purchase a Class II Biosafety Cabinet, CO2 incubator, centrifuge and a freezer. Moreover, the laboratory will be equipped with latest technologies instrument essential for organoids development and analysis, that will be acquired with other available resources, such as:
- Spark® Cyto 100: a multi-mode live-cell plate reader with real time fluorescence imaging and cytometry capabilities equipped with 4 color fluorescence, bright field and phase contrast imaging with 3 magnification levels and objectives. Full environmental control for temperature, gas and humidity protection will allow long term kinetic live cell assays to increase reproducibility.